INTRODUCTION: Recent data from the REMoDL-B trial show that addition of the proteasome inhibitor bortezomib to R-CHOP improves progression free survival (PFS) of patients (Pts) with activated B-cell (ABC) diffuse large B-cell lymphoma (DLBCL) compared to R-CHOP, but peripheral neuropathy limited dose intensity. Relative to bortezomib, carfilzomib (K) displays comparable clinical activity in plasma cell neoplasms but does not cause neurotoxicity. K has preferential clinical activity in relapsed non-germinal center (non-GC) DLBCL treated with chemotherapy (Torka, et al. Blood Adv. 2023). To explore the safety and efficacy of K in combination with R-CHOP (KR-CHOP), we performed a phase I trial to identify the recommended phase 2 dose (RP2D) in all DLBCL Pts followed by a phase II extension exclusively in non-GC DLBCL.
METHODS: Between 2015-2020, Pts with untreated de novo or transformed DLBCL, adequate organ function and ECOG PS ≤ 3 were enrolled at 3 US academic medical centers. As previously reported [Hill, et al, Blood (2018), 132 (Supl.1):1692], during phase I dose escalation, the RP2D of K was 20 mg/m 2 on days (D) 1 & 2 of cycle 1 and 56 mg/m 2 on D1 & 2 of cycles 2-6. Rituximab was given on D2 and CHOP was given on D3. All Pts received pegfilgrastim and zoster prophylaxis. To assess the cardiac safety of combining K with anthracycline, we compared echocardiogram data at baseline and conclusion of therapy from study Pts and control NHL Pts treated with CHOP. To gauge for differential clinical activity of KR-CHOP vs R-CHOP, we compared PFS and overall survival (OS) rates for study Pts with non-GC DLBCL vs. Pts with non-GC DLBCL receiving standard of-care (SOC) R-CHOP at the Cleveland Clinic during the same period. The outcomes were also compared between propensity score (PS)-matched pairs, to account for differences in baseline demographics and clinical risk.
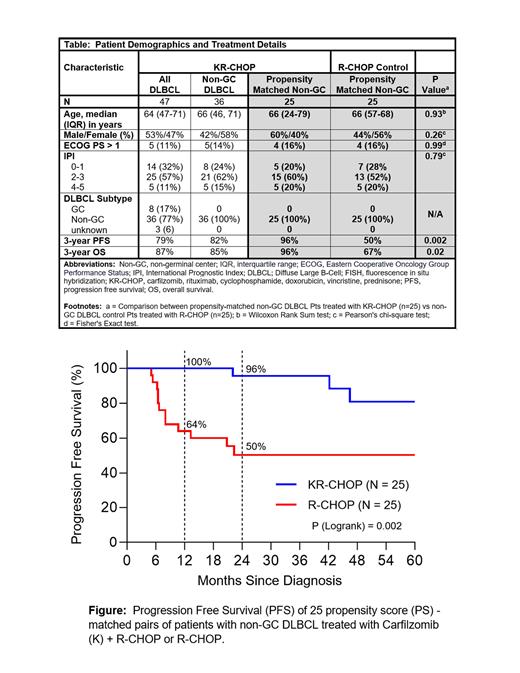
RESULTS: 48 Pts were enrolled, and 47 treated; 18 in dose levels < RP2D and 29 at RP2D.Baseline demographics and treatment outcomes are shown in the Table. All-grade (G) hematologic adverse events (AEs) were: anemia, 35% (22% ≤ G2, 13% G3); thrombocytopenia, 24% (15% ≤ G2, 2% G3, 7% G4); febrile neutropenia, 9% (all G3/G4). Non-hematologic AEs included: nausea, 49% (47% ≤ G2, 2% G3); vomiting, 23% (21% ≤ G2, 2% G3), peripheral sensory neuropathy, 27% (all ≤ G2); dyspnea, 24% (all ≤G2). There was one case of G3 congestive heart failure. Echocardiograms from 42 study Pts who completed 6 cycles of KR-CHOP showed no statistically significant difference in change in ejection fraction from baseline compared to 39 control Pts [95% vs. 94%, respectively (P = 0.59)]. The overall response rate was 89% (70% CR, 19% PR). At a median follow-up of 31 months, 3-year Kaplan-Meier estimates of PFS and OS for the study population were 79% and 87%, respectively. There were 8 deaths in the study: 4 due to lymphoma; 2 due to infection; 2 due to other causes. Comparing outcomes of the 36 study Pts with non-GC DLBCL to 172 control Pts receiving R-CHOP as SOC, more control Pts had stage IV disease (54% vs 34%, p=0.03) and older age (median 67 vs 66 years old, p=0.03), but less frequently >1 extranodal site of disease (23% vs. 42%, P = 0.02). PS matching using logistic regression identified 25 case-control pairs of Pts with non-GC DLBCL according to gender, age, and other International Prognostic Index (IPI) elements (see Table). In analysis of PS-matched pairs of Pts with non-GC DLBCL, Pts treated with KR-CHOP had better 3-year PFS and OS relative to Pts treated with R-CHOP as SOC [96% vs. 50% (p=0.002) (see Figure) and 67% vs. 96% (p = 0.02), respectively]. In Cox univariate analysis, treatment with R-CHOP vs. KR-CHOP was associated with an increased risk of PFS and OS with hazard ratios (HR) of 6.15 [95% confidence interval (CI) 1.71 - 22.05, P = 0.002] and 3.19 [(95% CI, 1.01 -10.11), P = 0.02], respectively.
CONCLUSION: KR-CHOP can be delivered safely, without excess cardiac toxicity or peripheral neuropathy. The superior PFS and OS rates for Pts with non-GC DLBCL vs. controls receiving R-CHOP are encouraging but should be interpreted with caution due to inherent selection bias in clinical trial populations. These data suggest that, like bortezomib, carfilzomib (K) may have preferential clinical activity in ABC DLBCL. Future randomized trials are justified using advanced molecular profiling to further investigate which Pts with DLBCL may benefit from addition of a proteasome inhibitor as part of frontline treatment.
OffLabel Disclosure:
Hill:Bristol Myers Squibb: Consultancy; BeiGene: Consultancy; AstraZeneca: Consultancy; Kite, a Gilead Company: Consultancy, Honoraria, Other: travel support, Research Funding; Pharmacyclics: Consultancy, Other: Advisory board, Research Funding; Genentech: Consultancy, Other: Advisory board, Research Funding; Gilead: Other: Advisory board; Incyte: Consultancy; AbbVie: Consultancy, Other: Advisory board, Research Funding. Torka:Genentech: Consultancy; Genmab: Consultancy; ADC Therapeutics: Consultancy; TG Therapeutics: Consultancy; Seagen: Consultancy; Lilly USA: Consultancy. Hernandez-Ilizaliturri:Dava Oncology: Consultancy; Incyte/Morphosys: Consultancy; BMS: Consultancy; Collectar: Consultancy; Gilead: Consultancy; ADC Therapeutics: Consultancy; Kite: Consultancy; Epizyme: Consultancy; AbbVie: Consultancy; Amgen: Consultancy; Novartis: Consultancy; BioGene: Consultancy. Jagadeesh:Debio Pharma: Research Funding; ATARA Biotherapeutics: Research Funding; MEI Pharma: Research Funding; LOXO Pharmaceuticals: Research Funding; Seagen: Research Funding; Trillium Pharmaceuticals: Research Funding; Regeneron Pharmaceuticals: Research Funding; AstraZeneca: Research Funding; Affimed: Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees. Winter:Janssen: Consultancy; Seattle Genetics: Consultancy; BeiGene: Consultancy; AstraZeneca: Consultancy; ADC Therapeutics: Consultancy. Caimi:SOBI: Honoraria; Novartis: Consultancy; BMS: Consultancy; Genentech: Consultancy; ADC Therapeutics: Consultancy; Lilly Oncology: Consultancy; Kite Pharma: Honoraria.
Carfilzomib is not approved for treatment of lymphoma.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal